Published online Dec 26, 2015. doi: 10.4330/wjc.v7.i12.824
Peer-review started: February 27, 2015
First decision: April 10, 2015
Revised: September 21, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: December 26, 2015
Processing time: 177 Days and 12.2 Hours
Despite repeated attempts to develop a unifying hypothesis that explains the clinical syndrome of heart failure (HF), no single conceptual paradigm for HF has withstood the test of time. The last model that has been developed, the neurohormonal model, has the great virtue of highlighting the role of the heart as an endocrine organ, as well as to shed some light on the key role on HF progression of neurohormones and peripheral organs and tissues beyond the heart itself. However, while survival in clinical trials based on neurohormonal antagonist drugs has improved, HF currently remains a lethal condition. At the borders of the neurohormonal model of HF, a partially unexplored path trough the maze of HF pathophysiology is represented by the feedback systems. There are several evidences, from both animal studies and humans reports, that the deregulation of baro-, ergo- and chemo-reflexes in HF patients elicits autonomic imbalance associated with parasympathetic withdrawal and increased adrenergic drive to the heart, thus fundamentally contributing to the evolution of the disease. Hence, on top of guideline-recommended medical therapy, mainly based on neurohormonal antagonisms, all visceral feedbacks have been recently considered in HF patients as additional potential therapeutic targets.
Core tip: At the borders of the neurohormonal model of heart failure (HF), a partially unexplored path trough the maze of HF pathophysiology is represented by the feedback systems. There are several evidences, from both animal studies and humans reports, that the deregulation of baro-, ergo- and chemo-reflexes in HF patients elicits autonomic imbalance associated with parasympathetic withdrawal and increased adrenergic drive to the heart, thus fundamentally contributing to the evolution of the disease. Hence, on top of guideline-recommended medical therapy mainly based on neurohormonal antagonisms, all visceral feedbacks have been recently considered in HF patients as additional potential therapeutic targets.
- Citation: Giannoni A, Mirizzi G, Aimo A, Emdin M, Passino C. Peripheral reflex feedbacks in chronic heart failure: Is it time for a direct treatment? World J Cardiol 2015; 7(12): 824-828
- URL: https://www.wjgnet.com/1949-8462/full/v7/i12/824.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i12.824
Heart failure (HF), a pathophysiological state in which the heart is unable to pump blood at a rate commensurate with the metabolizing tissues requirements, or can do so only with elevated filling pressures[1], is currently a real epidemic in western countries, affecting more than 20 million people in the world, with massive socio-sanitary costs[2].
Despite repeated attempts to develop a unifying hypothesis that explains the clinical syndrome of HF, no single conceptual paradigm for HF has withstood the test of time. The last model that has been developed, after the cardiorenal and the cardiocirculatory models focusing respectively on salt-water retention and low cardiac output/peripheral vasoconstriction, is the neurohormonal model[3]. This model has the great virtue of highlighting the role of the heart as an endocrine organ, as well as to shed some light on the key role on HF progression of neurohormones and peripheral organs and tissues beyond the heart itself. However, while survival in clinical trials based on neurohormonal antagonist drugs has improved, HF currently remains a lethal condition, with 50% mortality within 5 years of diagnosis and less than 15% survival after 10 years[2,4].
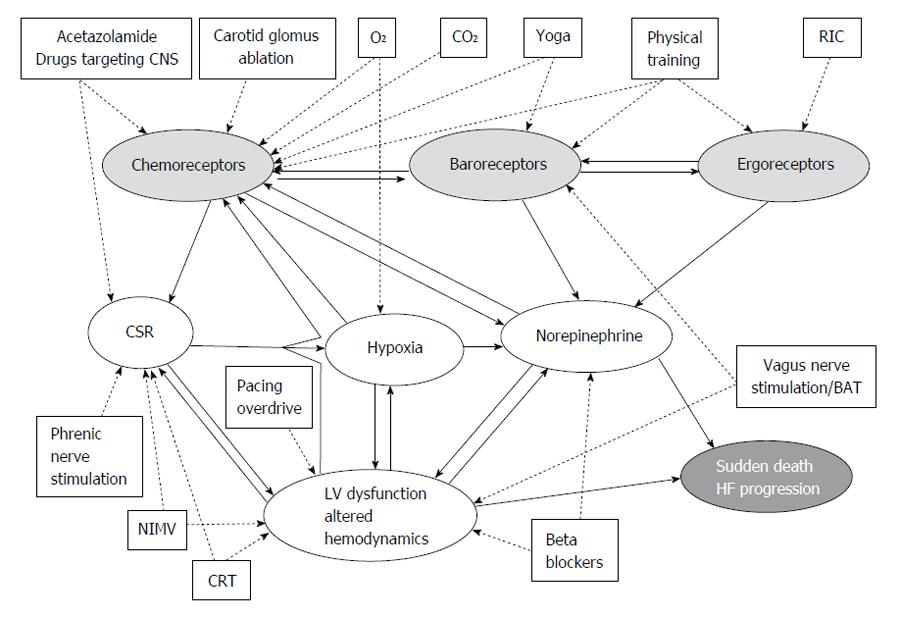
At the borders of the neurohormonal model, a partially unexplored path trough the maze of HF pathophysiology is represented by the feedback systems (Figure 1). There are indeed several evidences, from both animal studies and humans reports, that the deregulation of baro-, ergo- and chemo-reflexes in HF patients elicits autonomic imbalance associated with parasympathetic withdrawal and increased adrenergic drive to the heart, thus fundamentally contributing to worsening arrhythmias and haemodynamics. Hence, on top of guideline-recommended medical therapy mainly based on neurohormonal antagonisms, all visceral feedbacks have been recently considered in HF patients as additional therapeutic targets: baroreflex activation therapy for baroreceptors[5], physical training for muscle metaboreceptors[6], and carotid body (CB) denervation for chemoreceptors[7].
The baroceptors are mechanoreceptors located in the sinus caroticus and in the aortic arch, where terminal nerve endings are endowed in the wall of these vessels and activated by blood pressure-induced wall stretch. Information deriving from these sites travel along a path constituted by the nerve of Hering, that merges with the fibres of the glossopharingeal nerve; those travelling from the aortic arch take the path of the afferent fibres of the vagus nerve. Inputs hence travel towards the principal centre of integration of information regarding the cardiovascular system, that is the nucleus tractus solitarii in the dorsal area of its medial and lateral divisions. Here signal are processed and integrated with information ascending from the periphery and descending from central nervous system and given back to the heart and peripheral arterial vessels via the vagus nerve[8]. The response is a vagally-mediated change in heart rate and a sympathetic modulation of vasomotion, in order to preserve blood pressure stability over time and avoid fluctuations[9]. Altered baroreflex sensitivity (BRS) has been demonstrated to independently contribute to worsen prognosis in HF, mainly by failing to counteract the adrenergic activation with consequent electrical instability and arrhythmic sequelae, in both the pre- and post-betablocker era[10,11].
The baroreflex has been the first neurovegetative feedback to be clinically targeted in HF. BRS activation was first indirectly attempted by vagal nerve stimulation (VNS). After the first safety and tolerability reports on VNS (side effects: Hoarseness, cough and sensation of electrical stimulation) some preliminary studies also showed amelioration of symptoms and indexes of left ventricular (LV) remodelling[12,13]. These observations led to a phase III sham-controlled trial. The neural cardiac therapy for HF trial enrolled 87 patients with systolic HF [LV ejection fraction (LVEF) < 35%] who underwent device implantation and randomization to device in ON or OFF modality, but failed to demonstrate any effect of VNS on both primary (LV end systolic diameter) and secondary endpoints (LV end systolic volumes, LVEF, oxygen consumption and natriuretic peptide levels)[14].
Baroreceptor stimulation could also be achieved by directly stimulating carotid sinus by subcutaneously implanted device: This approach is known as baroreflex activation therapy (BAT). The first promising results obtained in an animal model of HF (dog with HF induced by microembolization) in terms of reverse remodelling, improved systolic function and amelioration of neurohormonal profile (reduced adrenergic activity), where secondarily confirmed also in a proof of concept study performed in humans, where an amelioration of symptoms was also observed[15]. Few on-going randomized studies are currently addressing the efficacy and therapeutic potential of baroreflex activation therapy in HF; in particular, the CVRx® Rheos® Diastolic Heart Failure Trial (clinicaltrial.org: NCT00718939) and the Rheos® HOPE4HF Trial (NCT00957073) will address the impact of BAT on diastolic HF (LVEF > 40%), whereas in systolic HF patients, the only ongoing randomized trial is the Barostim HOPE4HF (Hope for Heart Failure) study (NCT01720160).
The chemoreflex is physiologically in charge of proportionally modulating ventilation in response to a change in the respiratory gases, namely oxygen (O2) and carbon dioxide (CO2), in order to keep pH constant for enzymatic processes. Classical physiology indicates two separate chemoreceptor groups: Peripheral chemoreceptors (PC) located in carotid-aortic bodies and sensitive both to hypoxia and hypercapnia/acidosis, and central chemoreceptors (CC) located in different regions of the brainstem, cerebellum, hypothalamus and glia and considered to be sensitive only to hypercapnia/acidosis.
Chemoreceptors seem to act as primary inputs in HF. Several studies indicate that both PC and CC are hyperactive in HF[16-19]. The increased activity of chemoreceptors is commonly considered the main determinants of Cheyne-Stokes respiration[16-19], a detrimental respiratory pattern (with prognostic significance) characterized by alternating cycles of hyperventilation and apneas, with unfavourable oxygen desaturation. Furthermore, PC/CC hypersensitivity also negatively impact on respiration kinetics during exercise with ventilatory inefficiency and dyspnoea on effort in HF patients[18,19]. The hyperactivity of PC/CC, both directly (baseline tonic activity and phasic stimulation during O2/CO2 changes)[20] and indirectly, via Cheyne-Stokes respiration (CSR) occurrence[21], is also responsible of increased adrenergic drive and arrhythmias in HF patients[17-19]. Finally, increased chemosensitivity to both hypoxia[16] and hypercapnia[19] was found to be an independent prognostic marker in HF.
A partial inhibitory effect on PC was shown in HF patients with both transient hyperoxia, and drugs, such as dihydrocodeine or acetazolamide. In HF patients, dihydrocodeine mediated PC inhibition was only associated with improved exercise performance[22]. In the same setting, acetazolamide[23] and hyperoxia[24] were instead associated with about 50% reduction of CSR severity, translating in the case of hyperoxia also with reduced sympathetic activity. Denervation of the PC chemoreceptors by CB ablation in animals with experimentally induced HF has recently emerged as a very promising option. CB ablation is indeed able to normalize the chemoreflex sensitivity in HF animals, with reduction of both adrenergic activity and disappearance of central apneas[7,25]. This was confirmed also by pharmacologic attenuation of CB activity with an inhibitor of hydrogen sulfide[26]. Interestingly, in a model of HF induced by coronary ligation in rats, CB also reduced the amount of myocardial fibrosis unrelated to myocardial infarction, with positive effect on left ventricular systolic function and, more importantly on short term survival[25]. A single report in a patient with HF has testified the feasibility in humans[27]. Differently from these still preliminary, but intriguing results on PC modulation, currently no studies have tested the possibility to directly act on CC, maybe due to the multiplicity of CC centers in the central nervous system, the complexity of their interlink, and the difficulty to directly and selectively act on these receptors.
The ergoreflex is the neural mechanism enabling to modulate ventilation and sympathetic outflow according to the intensity of physical activity[28]. Its components are the metaboreflex, activated by the accumulation of metabolites in the exercising muscles, and the mechanoreflex, responsive to muscle tension during exercise[29-31].
HF patients frequently develop a skeletal myopathy ascribable to deconditioning, reduced perfusion of the muscles, inflammation, and a systemic catabolic state[29,30,32]. In 1994, a “muscle hypothesis” of HF was formulated, suggesting that ergoreceptor contribution to the autonomic, hemodynamic, and respiratory responses to exercise would be enhanced in CHF patients[33]. Two years later, ergoreflex overactivity was first found in HF patients compared with healthy subjects[6]. These results were corroborated by subsequent studies, which correlated increased ergoreceptor sensitivity to lower lean body mass, reduced exercise tolerance, decreased left ventricular function, and worse New York Heart Association functional class[30]. Interestingly, in HF patients with preserved exercise capacity, ergoreflex overactivity has been also associated with increased central and peripheral chemoreceptor sensitivity, and depressed baroreceptor sensitivity[30].
Currently the only acknowledged treatment for modulating ergoreflex overactivity is represented by exercise training. The effects of training on ergoreflex sensitivity have been evaluated mostly in animal models[34]. In humans, six weeks of forearm training were able to markedly reduce metaboreceptor sensitivity, while six weeks of detraining brought the situation back to baseline[29]. A positive effect on muscle structure and function has been after confirmed in other studies, still in HF patients[35,36]. It is reasonable to assume that the positive impact of exercise training on HF patients (in terms of increased exercise tolerance, quality of life, cardiac function, neuro-hormonal activation and overall prognosis)[37-40] partially relies upon reduced ergoreflex overactivity, as confirmed by a recent study[41].
The lessons learned from failures (e.g., inotropic drugs) and the successes (e.g., neurohormonal antagonist drugs) in treating HF indicate that the development of innovative treatments for HF should take into account the complex pathophysiology of the disease: In particular, new treatments should target the pathways involved in the evolution of the disease. As outlined above, peripheral reflexes are deeply involved in the pathophysiology of HF and represent a potential target of therapy. Although, some preliminary data in animals and humans are promising, more studies enrolling a large number of patients are clearly needed to reinforce the rationale of treating the peripheral reflex feedbacks and to disclose the prognostic value of these interventions.
P- Reviewer: Kataoka H, Ng TMH S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Colucci WS, Braunwald E. Pathophysiology of Heart Failure. A textbook of cardiovascular medicine. 7th ed. Pennsylvania, Philadelphia, US: Elseviewer Saunders 2005; 539. |
| 2. | Tendera M. The epidemiology of heart failure. J Renin Angiotensin Aldosterone Syst. 2004;5 Suppl 1:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 714] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2106] [Cited by in RCA: 2054] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 5. | Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA, Kieval RS. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 379] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Spyer KM. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994;474:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 332] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Cowley AW. Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231-300. [PubMed] |
| 10. | Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 303] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, Sleight P, Febo O. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009;53:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone-Marsan N, Tavazzi L. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 14. | Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2015;36:425-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 15. | Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, Lovett EG, Mancia G, Grassi G. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16:977-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 314] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Giannoni A, Emdin M, Poletti R, Bramanti F, Prontera C, Piepoli M, Passino C. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci (Lond). 2008;114:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, Piepoli M, Passino C. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53:1975-1980. [PubMed] |
| 20. | Hering D, Zdrojewski Z, Król E, Kara T, Kucharska W, Somers VK, Rutkowski B, Narkiewicz K. Tonic chemoreflex activation contributes to the elevated muscle sympathetic nerve activity in patients with chronic renal failure. J Hypertens. 2007;25:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | van de Borne P, Oren R, Abouassaly C, Anderson E, Somers VK. Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;81:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Chua TP, Harrington D, Ponikowski P, Webb-Peploe K, Poole-Wilson PA, Coats AJ. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 1997;29:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Fontana M, Emdin M, Giannoni A, Iudice G, Baruah R, Passino C. Effect of acetazolamide on chemosensitivity, Cheyne-Stokes respiration, and response to effort in patients with heart failure. Am J Cardiol. 2011;107:1675-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Staniforth AD, Kinnear WJ, Starling R, Hetmanski DJ, Cowley AJ. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62:2422-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Del Rio R, Marcus NJ, Schultz HD. Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol (1985). 2013;114:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Niewiński P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W. Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol. 2013;168:2506-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Piepoli MF, Coats AJ. The ‘skeletal muscle hypothesis in heart failure’ revised. Eur Heart J. 2013;34:486-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M, Jankowska EA, Anker SD, Capucci A, Banasiak W, Ponikowski P. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Piepoli MF, Coats AJ. Increased metaboreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol (1985). 2007;102:494-496; discussion 496-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Middlekauff HR, Sinoway LI. Increased mechanoreceptor stimulation explains the exaggerated exercise pressor reflex seen in heart failure. J Appl Physiol (1985). 2007;102:492-494; discussion 496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 381] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 33. | Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S36-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 224] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Wang HJ, Li YL, Zucker IH, Wang W. Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1260-R1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Adamopoulos S, Coats AJ, Brunotte F, Arnolda L, Meyer T, Thompson CH, Dunn JF, Stratton J, Kemp GJ, Radda GK. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol. 1993;21:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 250] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol. 2012;590:1243-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Piepoli MF. Exercise training in chronic heart failure: mechanisms and therapies. Neth Heart J. 2013;21:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol. 2006;47:1835-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 420] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 40. | Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 494] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 41. | Antunes-Correa LM, Nobre TS, Groehs RV, Alves MJ, Fernandes T, Couto GK, Rondon MU, Oliveira P, Lima M, Mathias W. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am J Physiol Heart Circ Physiol. 2014;307:H1655-H1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |